YOUR LIFE. YOUR JOURNEY. YOUR POTENTIAL.
A stress fracture occurs when abnormal and repetitive loading is applied on healthy bone. The body cannot adapt quickly enough, leading to microdamage and fracture of the bone.
Stress fractures are a common athletic injury, occurring in up to 40% of athletes at some point in their career. Stress fractures account for up to 10% of all orthopedic injuries and up to 20% of injuries seen in sports medicine clinics (Abbot et al, 2020; Changstrom et al, 2015). Studies suggest the annual incidence of stress fractures may be greater than 20% in runners (Tenforde et al, 2015; Bennell et al, 1996).
Stress fractures are most often found in the lower extremities (Kiel & Kaiser, 2020; Shapiro et al, 2020). The sites of stress fractures vary from sport to sport. Among track athletes, stress fractures of the navicular and metatarsal bones in the foot and the tibia in the leg are common. In distance runners, the tibia and fibula are common. In dancers, it can be the metatarsal bones which are most vulnerable. In the military, the calcaneus (heel) and metatarsal bones were the most commonly cited injuries, especially in new recruits, owing to the sudden increase in running and marching without adequate preparation (Barrack et al, 2014).
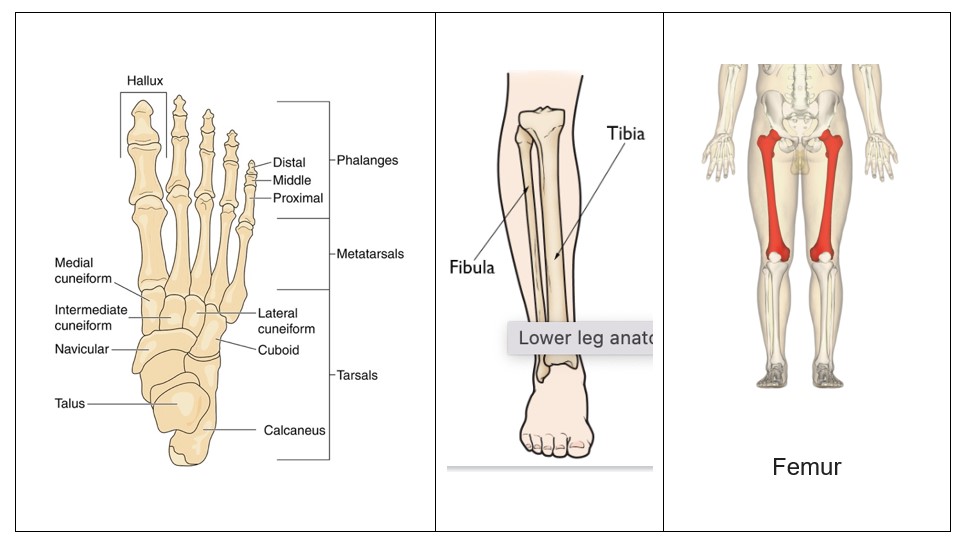
The order of prevalence or more importantly, the order of vulnerability for bone in the lower extremity is the tibia bone in the leg (49%), tarsal bones in the foot (25%), metatarsals (9%), femur, and fibula (Abbott et al, 2020; Asano et al, 2014). The ulna in the forearm is the bone most affected in the upper extremity.
The increased popularity of endurance running has contributed to the tibia (49% prevalence) replacing the metatarsals (9%) as the most common location for lower extremity SFx.
Bone
Stress fractures are a bone injury, and it is important to understand what makes healthy, strong bones that can withstand the stresses put on them during training and exercise.
-Bone Building
Bone mass is primarily created during childhood through physical activity and the appropriate nutrition of the elements needed to make strong bone. Physical activity during childhood is critical, as bone is a dynamic tissue that interacts with and responds to its environment. Physical activity creates a stress load and forces on bones through the contractile activity of muscles and through ground reaction forces (Lanyon et al, 1975; Usui et al, 2003). Ground reaction forces are exerted by the ground on the body as contact is made, and the force accelerates with movement.
During physical activity, when your muscles pull on your bones, it responds during the moment, while also starting a cascade of biochemical signals that will begin to strengthen those areas of the bone (Goodman et al, 2015). The harder the muscles tug, the more your body renews and strengthens those bones through increased calcium deposits; accordingly, bones become denser and stronger. Muscle forces themselves account for a significant majority of the load applied to the bone (Scott & Winter, 1990).
After childhood, bone mass decreases gradually through adulthood, making childhood a critical time to build bone that will sustain a lifetime of use. (Robling & Turner, 2009; Arfat et al, 2014; Klein-Nulend et al, 2013).
-Bone Remodeling
Bone constantly destroys and regenerates itself through a process termed remodeling. To fulfil this function, bone hosts two cell populations: osteoclasts, which break down bone tissue (called matrix) and then resorb mineralized bone matrix; and osteoblasts, which deposit new matrix, building new bone that eventually becomes mineralized. (Hadjidakis & Androulakis, 2006; Baron & Kneissel, 2013; Hiam et al, 2021).
This succession of destruction and formation occurs constantly throughout the skeleton. It takes 3-4 months for one remodeling cycle to complete the sequence of bone resorption, formation, and mineralization, and a minimum of 6-8 months to achieve a new steady-state bone mass that is measurable (Ducy, 2011).
The remodeling process is essential to have healthy bone, as it removes old or damaged bone, followed by the deposit of new bone material. Anything that interferes with remodeling would lead to weak or fragile bone, susceptible to injury.
While bone modeling occurs primarily in childhood, bone remodeling happens mainly during adulthood to remove micro-damaged and old bone, adapt bone tissue to mechanical loading, and maintain the strength and integrity of the skeleton (Sims & Martin, 2014).
Bone health is sustained if the osteoblast bone building activity is balanced with osteoclast resorption of old bone. If the balance is not maintained, such that there is not enough time for repair and remodeling, then bone health and strength can decline, and stress fractures can take place (Brennan et al, 2019).
Diet and Nutrition
The original risk for stress fractures is the health of your bones, and whether you have the diet and nutrition that produces healthy bones. As discussed, bone mass is created during childhood, and maintained through remodeling in adulthood. Strong bone results from physical activity, being outside, and the appropriate nutrition, such that you eat the foods that contain the nutrients for bone.
Essential nutrients for bones include vitamin K, vitamin D, and magnesium. This is discussed in detail on the Nutrition page. We will briefly summarize the importance of these nutrients, and encourage you to read about them in more detail at the above links.
Vitamin K is needed to activate proteins in bone cells, which leads to bone mineralization, bone formation, bone strength and density, preventing bone loss and reducing fractures. There is a wealth of research showing how vitamin K improves bone health, in a variety of ways. Insufficient intake of vitamin K means that these proteins remain inactive, which can lead to excess calcium building up in soft tissues, rather than in bone (Masterjohn, 2007; El Asmar et al, 2014). Low intake of vitamin K is associated with a higher risk of fracture (Ma et al, 2022).
There are two types of vitamin K for human consumption, K1 (phylloquinone) and K2 (menaquinones). K1 comes from green leafy vegetables, and K2 comes from fermented foods such as hard cheese, sauerkraut, and sausages, etc. And vitamin k works in synergy with vitamin D to regulate bone resorption, bone growth and bone distribution (Kidd, 2010).
Vitamin D is a fat-soluble vitamin that is naturally synthesized by the skin following exposure to the ultraviolet rays of the sun. Vitamin D plays a main role in regulating calcium metabolism by increasing intestinal calcium absorption from the food you eat (Bouillon, 2001; Holick, 2006; Fleet, 2017]. Vitamin D stimulates the production of the key bone proteins such as osteocalcin. (Gundberg et al, 2012). Research has found that Vitamin D stimulates bone formation, bone turnover (Foo et al, 2009), bone mass (Välimäki et al, 2004) and bone remodeling (Maimoun et al, 2008), and lowers the risk of fractures (Yakabe et al, 2023; Waterhouse et al, 2023), stress fractures in general (Tenford et al, 2010; Abbot et al, 2019), and specifically in the tibia and fibula (Burgi et al, 2011) and metatarsal (Knechtle et al, 2021).
Magnesium is one of the twelve minerals designated as an essential nutrient and its importance is hard to overstate. Magnesium plays a central role in processes such as protein synthesis, energy production, muscle contraction and relaxation, cardiac activity, and bone health, while also offering anti-inflammatory and antioxidant benefits (Laires & Monteiro, 2008; Barbagallo, 2010; Barbagallo et al, 2021). As such, magnesium is recognized for its critical role in athletic performance and overall health (Volpe, 2015). Mag has a mutually dependent relationship with vitamin D, and together with vitamin K, they form an effective cocktail for bone health and healing.
Before vitamin D can perform its vital functions, it must be converted to a biologically active form, first in the liver and then in the kidneys. The enzymes that do this conversion need Mag to function properly. A high consumption of Mag reduces the risks of vitamin D deficiency in the general population (Deng et al, 2013; Vázquez-Lorente, et al, 2020). The magnesium-vitamin D partnership isn’t a one-way street. Vitamin D can enhance intestinal absorption of Mag, allowing the mineral to be more efficiently used by the body for skeletal mineralization (Dusso, 2014; Lanske & Razzaque, 2007; Uwitonze & Razzaque, 2018; Erem et al, 2019). And magnesium coordinates with vitamin K. When vitamin K2 (MK4) is available, it inhibits the bone resorption caused by the Mag insufficiency, restoring bone remodeling and improving bone health (Amizuka et al, 2005).
In addition to what you should eat, there are items that should be avoided. Current western dietary trends involve highly processed foods where many nutrients have been processed out of the product. As well, soft drinks and colas are very popular. However, they contain a high content of phosphoric acid (phosphate) which is used to enhance flavor. The phosphoric acid interferes with calcium and magnesium absorption and results in a loss of magnesium and calcium from bone via the kidneys. Drinking large amounts of these beverages during childhood can reduce the mineral density in bones (Tucker et al, 2006; Ahn & Park, 2021).
Training
Typically, physical training is based upon the principles of progressive overload and adaptation, whereby an increasing training stimulus is applied over time to elicit physiological adaptations and improvements in performance (Van Someren & Howatson, 2011). The training schedule is comprised of training volume, intensity, and frequency over weeks, months, and seasons to maintain or improve physiological capabilities. This progression of training stimulus helps muscles adapt and develop strength, along with the bones.
A healthy bone's response to increased progressive, repetitive loads is to repair itself and continually re-establish its state of homeostasis, ultimately accelerating bone remodeling processes and strengthening itself against further insults (Bennell et al, 1999; Rizzone et al, 2017; Abbott et al, 2019).
Take the example of runners, who put great load upon their bodies and bones. Depending on their mechanics, the frequency of training, and the distance traveled, a male runner can strike the ground 1000 times during a 1600x meter race and a woman can strike the ground 1200 times during that same race. During a 5K those runners can strike that ground 3000 and 3600 times, respectively. Every time their foot hits the ground it is driving down with power, and receiving the force from the ground, called the ground reaction force (GRF). Every time your foot hits the ground, there is an equal force that pushes back up your leg. Research shows that depending on whether they run heel first, a 70 kg (155 pounds) person could be experiencing as much as 210 kg of GRF with each ground contact, or 3 times body weight. This is a tremendous load on the body, and depending on the training frequency, that bone can be loaded multiples times during a week. One can see how quickly the body could be overloaded into an injury such as a stress fracture.
Training involves multiple components that can be considered risk factors for bone stress fractures. Many of these risk factors relate to a lack of recovery following training such that the bones and supporting muscle structures are unable to adapt fast enough. This is likely the primary cause, though there are a variety of other variables that can also contribute to stress fractures.
Recovery
Recovery is the period between the end of one training session or competition and the beginning of another session. Recovery includes rest, refueling through nutrition, and rehydration, so that the regeneration and repair by your body from your training load can take place. It is one of the most important components of training and performance. (Kellmann et al, 2018).
It is important to remember that the adaptations needed to progress in training take place during recovery. It is only with adequate recovery that your performance and capabilities can progress (Bishop et al, 2008). Training creates the stress related opportunities that the body then repairs and consolidates during recovery. It is only with adequate recovery, that you actually improve your performance (van Someren et al, 2011).
As an example, the body heals itself during sleep. If you have inadequate sleep, or disturbances to sleep timing, or sleep duration, it can interrupt the bone turnover markers and other biological processes that take place during sleep. In turn, this can disrupt the balance between bone resorption and formation, decreasing bone health and increasing fracture risk (Swanson et al, 2018). A lack of adequate recovery can compromise your immune system, lead to exhaustion, and can cause chronic joint and muscle pain.
In a study of 95 endurance sport athletes who self-reported their sleep time over a 2-week period, those who slept <7 h/day were at a 51% increased risk of new injury, whereas athletes who slept >7 h/day reduced new injury risk by 37% (Johnston et al, 2020). Specific to bone stress fractures, in a population of 314 adolescent high school athletes who experienced 346 stress fractures over a 2-year period, those who reported sleeping less had more stress fractures (7.2 vs. 7.95 h/day) (Nussbaum et al, 2019).
Periodization refers to physical training that is structured around periods of progressively-loaded training stress that are then followed by rest. Planning recovery within and across training or competition cycles is necessary and should consider the characteristics of individual training sessions (e.g., metabolic demand), individual variations in the recovery process, and desired physical adaptations (Nanclerio et al, 2022). Time away from the sport/training environment, rest, social recovery, and downtime are all important factors that need to be considered in the overall periodized plan. Insufficient recovery will not only impact an athlete’s performance in subsequent training bouts but will also curtail the potential physiological adaptations from the initial training bout and thereby fail to meet the basic purpose of the training process (Van Someren et al, 2010).
How much time recovery takes after a workout depends on several factors. For example, the intensity and nature of your training is a factor. Muscle recovery after strength training is more intensive and therefore takes longer than muscle recovery after running or after cycling, because more muscle tears occur during strength training. This can not only lead to soreness and pain, but the damage can also inhibit your muscles’ ability to replenish their stores of glycogen — the primary fuel they use for energy.
In cardio or endurance sports, including running and cycling, your muscle fibers need to pull together less to perform the required movement. In addition to the type of effort you have made, the post-treatment is also very important to enhance your muscle recovery. Depending on your training and the measures you take after it, the recovery of your muscle fibers usually takes between 42 and 72 hours. Note: If you start training intensively again before you have recovered, you run the risk of muscle and tendon injuries.
It is well accepted that progressive over-load is necessary for improvement. However, it is important to find the balance between over-load versus overtraining, which is training with only limited recovery. Overtraining represents a chronic imbalance between training and recovery, which usually manifesting as a prolonged performance plateau or even a decline, though other symptoms can be apparent (Weakley et al, 2022). Overtraining is usually thought of strictly in terms of training, yet overtraining might also be expressed as under-recovering.
Jonathan Ross, a highly recognized and respected trainer from Baltimore, advises his clients that if they’re “hitting it hard,” then they need to devote equal time to “quitting it hard” to appropriately recover.
-Load
An important risk factor for stress fractures is training load. Generally speaking, overuse injuries occur when running intensity and/or volume increases at a pace the body cannot accommodate (Hreljac, 2005; Bertelsen et al, 2017). While it is unclear as to exactly how much is too much, rapid increases in training loads have been associated with increased risk of injury, including bone stress fractures (Rauh, 2014; Damsted et al, 2018). The danger is amplified if running mechanics are off.
Clinically, the bone's response to loading occurs along a continuum from normal remodeling to accelerated remodeling, stress reaction, stress injury, stress fracture, and finally complete fracture (Bennell et al, 1999; Barrack et al, 2014; Kraus et al, 2019). A healthy bone's response to increased repetitive loads is to repair itself and re-establish its state of homeostasis (Bennell et al, 1999; Abbott et al, 2019). However, if the bone's energy/nutrient stores are insufficient and the rest and recovery process is unable to keep pace with the repetitive loads, particularly at sites where stress is concentrated, the bone will fatigue, and stress fractures will develop and can progress in severity (Bennell et al, 1999; Miller & Best, 2016; Rizzone et al, 2017).
Training loads can be modified not only by intrinsic factors but also by extrinsic factors, such as footwear, surface, number of strides/cycles, magnitude of load (kinetics), and distribution of load (kinematics) (Bertelsen et al, 2017; Paquette et al, 2020). But the elements are complex, and it has been difficult to clarify any single element as preventing or promoting stress fractures.
Theoretically, footwear (shoes, orthotics) can attenuate force and/or influence kinematics, but the research on the ability of footwear to decrease stress fractures risk is unclear (Warden et al, 2014). While some have claimed that increased cushioning can protect from increased bone loads, others have claimed that minimalist shoes are the answer by reducing vertical loading rates (Rixe et a, 2012: Warne & Gruber, 2017). There is currently there is little evidence to support that any type of footwear can prevent running-related injuries, let alone bone stress injuries (BSIs) (Napier & Willy, 2018). However, there are advocates who recommend using shoes that help promote good running mechanics, such as shoes that help you to run on the front third of your foot.
Similarly, it is thought that a harder training surface increases loading relative to a softer one and thus may be a risk factor for BSIs, but the training surface and BSI risk relationship remains unclear and complex. For instance, one must also consider variables, such as leg stiffness, running distance, and surface accommodation, among many other variables, when determining the role of surface type on BSIs (Warden et al, 2014). Runners can modulate leg stiffness depending on running surface, which may mitigate the effect of hard surfaces or cushioned shoes (Ferris et al, 1998, 1999).
Again, stress fractures may result from changes in training, including gear, that are too abrupt for the bones to adapt to.
-Muscle Recruitment
One of the major roles of muscles during activity is energy absorption which attenuates and absorbs force (Paul et al, 1978: Bennell et al, 1999). This means that muscle development and recruitment are important components of any training plan.
When muscles become fatigued, they transmit greater energy to the surrounding bone. Thus, it is hypothesized, that the relatively lesser muscle and bone mass of the lower leg, particularly around the tibia, may explain the greater frequency of tibial stress fractures. (Garret et al, 1987) An athlete whose physiological capacity is suboptimal relative to the training load being applied could be at risk for bone stress. (Hadid et al, 2018). It is important to ensure that your muscle capacity, in the context of your training, is also developed to be sufficient.
-Biomechanics
Biomechanics are the science of movement of a living body, including muscles, bones, tendon, and ligaments working together to move. In short, it's the study and analysis of how all the individual parts of your body work together to make up athletic and everyday movements.
Abnormal biomechanics have been hypothesized to contribute to the development of stress fractures (Miller & Best, 2016) by subjecting the bone to loads it cannot withstand. Inappropriate posture and the wrong use of body mechanics prevents the proper utilization of ground reaction forces. The high impact of vertical component of ground force reactions must be absorbed by correct, balanced body mechanics (proper ground striking pattern and posture) for safe running.
Though research thus far points to increased vertical loading rate, hip adduction, and rearfoot eversion as key kinetic and kinematic risk factors for the development of stress fractures, it is important to note that the studies referenced in this section are retrospective in nature (Milner et al, 2010; Pohl et al, 2010).
Proper biomechanics are very individualized, reflective of a person’s body form, skeletal structure, limb length, etc. The literature does agree that running with a foot strike on the front of the foot is associated with fewer bone injuries as it allows the ankle joint to absorb impact (Daoud et al, 2012)
-Periodization
Type and frequency of activities are important risk factors for stress fracture. For example, repeated submaximal stresses (running, jumping, or marching); high-impact loading, new or excessive exercise, change in the type or intensity of the activity, and limited rest following excessive physical activity create risk. Runners with stress fractures exercised 3 more hours per week compared to runners who did not have SF, and dancers who practiced 6 or more hours per day had more SFs (Saunier & Chapurlat, 2018; Abbott & Bird, 2020; Moreira & Bilezikian, 2017)
-Change in Training/Equipment
Several studies have noted that an alteration in an athlete’s training program is one of the most significant factors resulting in an a stress injury Fredericson et al, 2006; James & Bates, 1978; Jones & Harris, 1989; Johansson, 1992). Whether it be a sudden increase in mileage, pace, volume, or cross-training activity, anything that has been newly inserted into the program requires adequate time for physiologic adaptation to accommodate the new forces. It is when there is not time to adjust that risk for injury increases.
-Prior Stress Fracture
Perhaps the strongest non-modifiable risk factor is prior bone injury. In a prospective study of female cross-country runners, those with a previous stress fracture had more than a five-fold higher rate of other bone injuries, particularly stress fractures, during the average 1.85-year follow-up than females without such a history (Kelsey et al, 2007). Similarly, in a prospective study of female and male high school runners, females had a six-fold and males a seven-fold increased risk of the development of a stress fracture if they had a stress fracture history (Tenforde et al, 2013).
Summary
Stress fractures are often cumulative and multifactorial (Nattiv et a, 2007; Kraus et al, 2019). Training load and recovery are key factors in the development of BSIs since it is the loading of the bone without sufficient recovery time for remodeling that results in a BSI.
References
Abbott A, Bird M, Wild E. Brown SM, Stewart G, Mulcahey MK. Part I: epidemiology and risk factors for stress fractures in female athletes. Phys Sportsmed. 2019;48;17–24.
Abbott A, Bird M, Brown SM, Wild EM, Stewart G, Mulcahey MK. Part II: presentation, diagnosis, classification, treatment, and prevention of stress fractures in female athletes. Phys Sportsmed. 2020 Feb;48(1):25-32.
Ahn H, Park YK. Sugar-sweetened beverage consumption and bone health: a systematic review and meta-analysis. Nutr U. 2012 May 5;20(1):41. Doi: 10.1186/s12937-021-00698-1.
Arfat Y, Xiao W-Z, Iftikhar S, Zhao F, Li D-J, Sun Y-L, et al. Physiological effects of microgravity on bone cells. Calcif Tissue Int. 2014 Jun;94(6):569-79.
Amizuka N, Li M, Maeda T. The interplay of magnesium and vitamin K2 on bone mineralization. Clin Calcium. 2005 Jul;15(7):57-61.
Asano LY, Duarte A, Jr, Silva AP. Brazilian Medical Association. Stress fractures in the foot and ankle of athletes. Rev Assoc Med Bras (1992). 2014;60(6):512–517.
Barbagallo M, Dominguez LJ, Galioto A, Pineo A, Belvedere M. Oral magnesium supplementation improves vascular function in elderly diabetic patients. Magnes Res. 2010 Sep;23(3):131-7.
Barbagallo M, Veronese N, Dominguez LJ. Magnesium in aging, health and diseases. Nutrients. 2021;13(2):463.https://doi.org/10.3390/nu13020463
Baron R, Keissel M. WNT Signaling in Bone Homeostasis and Disease: From Human Mutations to Treatments. Nature Medicine. 2013;19:179-192.
Barrack MT, Gibbs JC, De Souza MJ, Williams NI, Nichols JF, Rauh MJ, et al. Higher incidence of bone stress injuries with increasing female athlete triad-related risk factors: a prospective multisite study of exercising girls and women. Am J Sports Med. 2014;42:949–958.
Bennell KL, Malcolm SA, Thomas SA, Reid SJ, Brukner PD, Ebeling PR et al. Risk factors for stress fractures in track and field athletes. A twelve-month prospective study. Am J Sports Med. 1996 Nov-Dec;24(6):810-8.
Bennell K, Matheson G, Meeuwisse W, Brukner P. Risk factors for stress fractures. Sports Med. 1999;28:91–122.
Bertelsen ML, Hulme A, Petersen J, Brund R. K, Sørensen H, Finch C., et al. A framework for the etiology of running-related injuries. Scan J Med Sci Sports. 2017;27:1170–1180.
Bishop PA, Woods E, Krista A. Recovery from training: a brief review. J Strength & Conditioning Res. 2008 May;22(3):1015-1024.
Bouillon R. Vitamin D: photosynthesis, metabolism, and action to clinical applications. In: Degroot L, Jameson, JL, Burger HG, Eds., Endocrinology, 3rd Edition, WB Saunders, Philadelphia. 2001. p. 1009-1028.
Brennan M, O’Shea PM, O’Keeffe ST, Mulkerrin EC. Spontaneous insufficiency fractures. J Nutr. Health Aging. 2019;23:758–760.
Burgi AA, Gorham ED, Garland CF, Mohr SB, Garland FC, Zeng K, et al. High serum 25-hydroxyvitamin D is associated with a low incidence of stress fractures. J Bone Min Res. 2011;26:2371–2377.
Changstrom BG, Brou L, Khodaee M, Braund C, Comstock RD. Epidemiology of stress fracture injuries among US high school athletes, 2005-2006 through 2012-2013. Am J Sports Med. 2015;43(1):26–33.
Damsted C, Glad S, Nielsen RO, Sørensen H, Malisoux L. (2018). Is there evidence for an association between changes in training load and running-related injuries? A systematic review. Int J Sports Phys Ther. 2018;13:931–942.
Daoud AK, Geissler GJ, Wang F, Saretsky J, Lieberman DE. Foot strike and injury rates in endurance runners: a retrospective study. 2012 Jul;44(7):1325-34.
Deng X, Song Y, Manson JE, Signore LB, Zhang SM, Shrubsole MJ, et al. Magnesium, vitamin D status and mortality: results from US National Health and Nutrition Examination Survey (NHANES) 2001 to 2006 and NHANES III. BMC Medicine. 2013 Aug 2013;11:187(2013).
Driller M, Leabeater A. Fundamentals or icing on top of the cake? A narrative review of recovery strategies and devices for athletes. Sports. 2023;11(11):213. https://doi.org/10.3390/sports11110213
Ducy P. The role of osteocalcin in the endocrine cross-talk between bone remodeling and energy metabolism. Diabetologia. 2011 Jun;54(6):1291-7.
Dusso AS. Update on the biologic role of the vitamin D endocrine system. Curr Vasc Pharmacol. 2014 Mar;12(2):272-7.
El Asmar MS, Naoum JJ, Arbid EJ. Vitamin K dependent proteins and the role of vitamin K2 in the modulation of vascular calcification: A review. Oman Med J. 2014;29:172–177.
Erem S, Atfi A, Razzaque MS. Anabolic effects of vitamin D and magnesium in aging bone. J Steroid Biochem & Mol Biology. 2019;193:105400
Ferris DP, Louie M, Farley CT. Running in the real world: adjusting leg stiffness for different surfaces. Proc Biol Sci. 1998;265, 989–994.
Ferris DP, Liang K, Farley CT. Runners adjust leg stiffness for their first step on a new running surface.J Biomech. 1999;32:787–794.
Fleet JC. The role of vitamin D in the endocrinology controlling calcium homeostasis. Mol Cell Endocrinol. 2017 Sep 15;453:36-45.
Foot LH, Zhang Q, Zhu K, Ma G, Hu X, Greenfield H, et al. Low vitamin D status has an adverse influence on bone mass, bone turnover, and muscle strength in Chinese adolescent girls. J Nutri. 2009 May;139(5):1002-1007.
Fredericson M, Jennings F, Beaulieu C, Matheson GO. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17(5):309-325.
Garrett Jr, WE, Nikolaou PK, Seaber AV, Glisson RR, Ribbeck BM. The effect of muscle architecture on the biomechanical failure properties of skeletal muscle under passive extension. Am J Sports Med. 1988 Jan;16(1): https://doi.org/10.1177/0363546588016001
Garrett Jr, WE, Safran MR, Seaber AV, Glisson RR, Ribbeck BM. Biomechanical comparison of stimulated and nonstimulated skeletal muscle pulled to failure. Am J Sports Med. 1987 Sep-Oct;15(5):448054.
Goodman CA, Hornberger TA, Robling AG. Bone and skeletal muscle: key players in mechanotransduction and potential overlapping mechanisms. Bone. 2015;80:24–36.
Gundberg CM., Lian JB., Booth SL. Vitamin k-dependent carboxylation of osteocalcin: Friend or foe? Adv Nutr. 2012;3:149–157.
Hadjidakis DJ, Androulakis II. Bone remodeling. Ann N Y Acad Sci. 2006 Dec:1092:385-96.
Hadid A, Epstein Y, Shabshin N, Gefen A. Biomechanical model for stress fracture-related factors in athletes and soldiers. Med Sci Sports Exerc. 2018;50:1827–1836.
Hamstra-Wright KL, Bliven KCH, Napier C. Training load capacity, cumulative risk, and bone stress injuries: a narrative review of a holistic approach. Front Sports Act Living. 2021;3:665683.
Hiam D, Voisin S, Yan XU, Landen S, Jacques M, Papadimitriou ID, et al. The association between bone mineral density gene variants and osteocalcin at baseline, and in response to exercise: The gene SMART study. Bone. 2019 Jun;123:23-27.
Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006 Mar;81(3):353-73.
Kidd PM. Vitamins D and K as pleiotropic nutrients: Clinical importance to the skeletal and cardiovascular systems and preliminary evidence for synergy. Altern Me. Re. A J Clin. 2010;15:199–222.
Hreljac A. Etiology, prevention, and early intervention of overuse injuries in runners: a biomechanical perspective. Phys Me. Rehabil Clin N Am. 2005;16:651, vi–667, vi. 10.1016/j.pmr.2005.02.002
James SL, Bates BT, Osternig LR. Injuries to runners. Am J Sports Med. 1978 Mar-Apr;6(20);40-50.
Johansson C, Ekenman I, Lewander R. Stress fracture of the tibia in athletes: diagnosis and natural course. Scandin J Med & Scie in Sports. 1992 Jun; https://doi.org/10.1111/j.1600-0838.1992.tb00326.x
Johnston R, Cahalan R, Bonnett L, Maguire M, Glasgow P, Madigan S, et al. General health complaints and sleep associated with new injury within an endurance sporting population: a prospective study. J Sci Med Sport. 2020;23:252–257.
Jones BH, Harris JMA, Vinh TN, Rubin C. Exercise-induced stress fractures and stress reactions of bone: epidemiology, etiology, and classification. Exercise & Sport Sci Reviews. 1989 Jan;17(1):379-422.
Kellmann M, Bertollo M, Bosquet L, Brink M, Coutts AJ, Duffield R, et al. Recovery and performance in sport: consensus statement. Int J Sports Physiol Perform. 2018;13:240–245.
Kelsey J, Bachrach L, Procter-Gray E, Nieves J, Greendale G, Sowers M, et al. Risk factors for stress fracture among young female cross-country runners. Med Sc. Sports Exerc. 2007;39:1457–1463.
Kiel J, Kaiser K. Stress Reaction and Fractures. StatPearls Publishing; Treasure Island, FL, USA: 2020.
Klein-Nulend J, Bakker AD, Bacabac RG, Vatsa A, Weinbaum S. Mechanosensation and transduction in osteocytes. Bone. 2013 Jun;54(2):182-90.
Knechtle B, Nikolaidis PT, Lutz B, Rosemann T, Baerlocher CB. Pathologic fracture of the thoracic spine in a male master ultra-marathoner due to the combination of a vertebral hemangioma and osteopenia. Medicina. 2017;53:131–137.
Kraus E, Tenforde AS, Nattiv A, Sainani KL, Kussman A, Deakins-Roche M, et al. Bone stress injuries in male distance runners: higher modified Female Athlete Triad Cumulative Risk Assessment scores predict increased rates of injury. Br J Sports Med. 2019;53:237–242.
Laires MJ, Monteiro C. Exercise, magnesium and immune function. Magnes Res. 2008 Jun;21(2):92-6.
Lanske B, Razzaque MS. Vitamin D and aging: old concepts and new insights. J Nutr Biochem. 2007 Dec;18(12):771-7.
Lanyon LE, Rubin CT. Static vs dynamic loads as an influence on bone remodeling. J Biomechanics. 1984;17(2):897-905.
Ma M-L, Ma Z-j, He Y-l, Sun H. Efficacy of vitamin K2 in the prevention and treatment of postmenopausal osteoporosis: A systematic review and meta-analysis of randomized controlled trials. Front Public Health. 2022 Aug 10;10 979649
Maïmoun L, Sultan C. Effects of physical activity on bone remodeling. Sci Direct. 2011;60:373-388.
Masterjohn C. Vitamin D toxicity redefined: Vitamin K and the molecular mechanism. Med. Hypotheses. 2007;68:1026–1034.
Miller TL, Best TM. Taking a holistic approach to managing difficult stress fractures. J Orthop Surg Res. 2016;11:98. 10.1186/s13018-016-0431-9
Milner C, Hamill J, Davis IS. Distinct hip and rearfoot kinematics in female runners with a history of tibial stress fracture. J Orthop Sports Phys Ther. 2010;40:59–66. 10.2519/jospt.2010.3024
Moreira CA, Bilezikian JP. Stress Fractures: Concepts and Therapeutics. J Clin Endocrinol Metab. 2017;102(2):525–534.
Nanclerio F, Moody J, Chapman M. Applied periodisation: a methodological approach. J Hum Sport Exerc. 2022;8:350–366.
Napier C, Willy RW. Logical fallacies in the running shoe debate: let the evidence guide prescription. Br J Sports Med. 2018;52:1552–1553.
Nattiv A, Loucks AB., Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP, et al. American college of sports medicine position stand. The female athlete triad. Med Sci Sports Exerc. 2007;39:1867–1882.
Nussbaum ED, Bjornaraa J, Gatt CJ. Identifying factors that contribute to adolescent bony stress injury in secondary school athletes: a comparative analysis with a healthy athletic control group. Sports Health. 2019;11:375–379.
Paquette MR, Napier C, Willy RW, Stellingwerff T. Moving beyond weekly “distance”: optimizing quantification of training load in runners. J Orthop Sports Phys. Ther. 2020;50:564–569.
Paul IL, Munro M, Abernethy PJ, Simon SR, Radin EL. Rose RM. Musculo-skeletal shock absorption: relative contribution of bone and soft tissues at various frequencies. J Biomech. 1978;11:237–239.
Pohl MB, Lloyd C, Ferber R. Can the reliability of three-dimensional running kinematics be improved using functional joint methodology? Gait Posture. 2010;32, 559–563.
Rauh MJ. Summer training factors and risk of musculoskeletal injury among high school cross-country runners. J Orthop Sports Phys Ther. 2014;44:793–804.
Rixe JA, Gallo RA, Silvis ML. The barefoot debate: can minimalist shoes reduce running-related injuries? Curr Sports Med. Rep. 2012;11:160–165.
Rizzone KH, Ackerman KE, Roos KG, Dompier TP, Kerr ZY. (2017). The Epidemiology of stress fractures in collegiate student-athletes, 2004-2005 through 2013-2014 academic years. J Athletic Train. 2017;52:966–975.
Robling AG, Turner CH. Mechanical signaling in bone modeling and remodeling. Crit Rev Eukaryot Gene Expr. 2009;19(4):319-38.
Saunier J, Chapurlat R. Stress fracture in athletes. Joint Bone Spine. 2018;85(3):307–310.
Scott SH, Winter DA. Internal forces of chronic running injury sites. Med Sci Sports Exerc. 1990;22:357–369.
Shapiro M, Zubkov K, Landau R. Diagnosis of Stress fractures in military trainees: A large-scale cohort. BMJ Mil Health. 2020;2020:001406. doi: 10.1136/bmjmilitary-2020-001406.
Sims NA, Martin TJ. Couple the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. Bonekey Rep. 2014;3:481.
Swanson CM, Kohrt WM, Buxton OM, Everson CA, Wright KP, Orwoll ES, et al.The importance of the circadian system and sleep for bone health. Metabolism. 2018;84:28–43.
Tenforde AS, Barrack MT, Nattiv A, Fredericson M. Parallels with the female athlete triad in male athletes. Sports Med. 2016; Feb;46(2):171-82.
Tenforde AS, Sayres LC, Sainani KL, Fredericson M. Association between serum 25(OH)D concentrations and bone stress fractures in Finnish young men. PmR. 2010;2:945–949.
Tenforde AS, Sayres LC, McCurdy ML, Sainani KL, Fredericson M. Identifying sex-specific risk factors for stress fractures in adolescent runners. Med Sci Sports Exerc. 2013;45:1843–1851.
Tucker KL, Morita K, Qiag N, Hannan MT, Adrienne CL, Kiel DP. Colas, but not other carbonated beverages, are associated with low bone mineral density in older women: the Framingham Osteoporosis Study. Am J Clin Nutr. 2006 Oct;84(4):936-42.
Usui T, Maki K, Toki Y, Shibasaki Y, Takanobu H, Takanishi A, et al. Measurement of mechanical strain on mandibular surface with mastication robot: influence of muscle loading direction and magnitude. Orthod Craniofac Res. 2003;6:163–167.
Uwitonze AN, Razzaque MS. Role of magnesium in vitamin D activation and function. J Am Osteophath Assoc. 2018 Mar 1;118(3):181-189.
Välimäki V-V, Alfthan H, Lehmuskallio E, Löyttyniemi E, Sahi T, Stenman U-H, et al. Vitamin d status as a determinant of peak bone mass in young Finnish men. J Clin Endocrin & Metab. 2004 Jan;89(1):76-80.
van Someren K, Howatson G. Training, recovery and adaptation. In Sports Injuries; Oxford University Press: Oxford, NY, USA. 2011. p. 83–88.
Vázquez-Lorente H, Herrera-Quintana L, Molina-Lopez J, Gamarra-Morales Y, López-González B, et al. Response of vitamin D after magnesium intervention in a postmenopausal population from the province of Granada, Spain. Nutrients. 2020 Aug;12(8):2283.
Volpe SL. Magnesium and the athlete. Curr Sports Med Rep. 2015 Jul-Aug;14(4):279-83.
Warden SJ, Davis IS, Fredericson M. Management and prevention of bone stress injuries in long-distance runners. J Orthop Sports Phys Ther. 2014:44,749–765.
Warne JP, Gruber AH. Transitioning to minimal footwear: a systematic review of methods and future clinical recommendations. Sports Med. 2017;3;33(2017).
Waterhouse M, Ebeling PR, McLeod DSA, English D, Romero BD, Baxter C, et al. The effect of monthly vitamin D supplementation on fractures: a tertiary outcome from the population-based, double-blind, randomized, placebo-controlled D-Health trial. Lancet Diabetes Endocrinol. 2023 May;11(4):324-332.
Weakley J, Halson SL, Mujika I. Overtraining Syndrome Symptoms and Diagnosis in Athletes: Where Is the Research? A Systematic Review. Int J Sports Physiol Perform. 2022;17:675–681.
Yakabe M, Hosoi T, Matsumoto S, Fujimori K, Tamaki J, Nakatoh S, et al. Prescription of vitamin D was associated with a lower incidence of hip fractures. Nature Sci Reports. 2023 Aug 9;13:12889.
Becoming an Uncommon Man with Ultra K
Meet Isaiah, a dedicated member of the 1st Ranger Battalion in Savannah, Georgia. His incredible journey not only reflects his commitment to becoming a state record holder and champion in track and field but also showcases his remarkable path to becoming an Army Ranger. Discover how he harnessed all the essential elements of Ultra K to achieve these extraordinary feats.

Manufactured and Distributed by:Koncentrated K, Inc.
P.O. Box 343, Manistique, MI 49854
www.UltraKVitamins.com
Copyright © 2023 - 2025 Koncentrated K, All rights reserved. Site Map